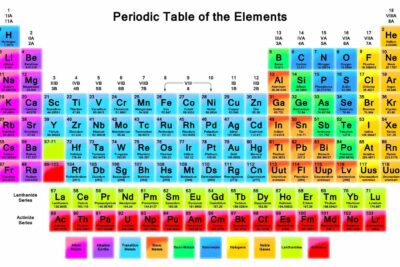
Most of the chemical elements you encounter every day are combined with other elements to form compounds. Here's a gallery of pictures of the pure elements, so you can see what they look like.
The elements are listed in the order in which they appear in the periodic table; the first elements have the lowest atomic number, which increases through the table. Toward the end of the periodic table, there aren't any images of elements. Some are so rare only a few atoms have ever been produced, plus they are highly radioactive, so they often vanish an instant after creation. Yet, many elements are stable. Here's your chance to get to know them.
Lectura relacionada:
 Datos del Samario - Sm o Elemento 62
Datos del Samario - Sm o Elemento 62- Hydrogen - Element 1
- Helium - Element 2
- Lithium - Element 3
- Beryllium - Element 4
- Boron - Element 5
- Carbon - Element #6
- Nitrogen - Element 7
- Oxygen - Element #8
- Fluorine - Element 9
- Neon - Element 10
- Sodium - Element 11
- Magnesium - Element 12
- Aluminum - Element 13
- Silicon - Element 14
- Phosphorus - Element 15
- Sulfur - Element 16
- Chlorine - Element 17
- Argon - Element 18
- Potassium - Element 19
- Calcium - Element 20
- Scandium - Element 21
- Titanium - Element 22
- Vanadium - Element 23
- Chromium - Element 24
- Manganese - Element 25
- Iron - Element 26
- Cobalt - Element 27
- Nickel - Element 28
- Copper - Element 29
- Zinc - Element 30
- Gallium - Element 31
- Germanium - Element 32
- Arsenic - Element 33
- Selenium - Element 34
- Bromine - Element 35
- Krypton - Element 36
- Rubidium - Element 37
- Strontium - Element 38
- Yttrium - Element 39
- Zirconium - Element 40
- Niobium - Element 41
- Molybdenum - Element 42
- Ruthenium - Element 44
- Rhodium - Element 45
- Silver - Element 47
- Cadmium - Element 48
- Indium - Element 49
- Tin - Element 50
- Tellurium - Element 52
- Iodine - Element 53
- Xenon - Element 54
- Europium - Element 63
- Thulium - Element 69
- Lutetium - Element 71
- Tantalum - Element 73
- Tungsten - Element 74
- Osmium - Element 76
- Platinum - Element 78
- Gold - Element 79
- Mercury - Element 80
- Thallium - Element 81
- Lead - Element 82
- Bismuth - Element 83
- Uranium - Element 92
- Plutonium - Element 94
Hydrogen - Element 1
Stars and this nebula consist mainly of the element hydrogen.
NASA/CXC/ASU/J. Hester et al., HST/ASU/J. Hester et al.
Hydrogen is the first element on the periodic table, with 1 proton per atom. It's the most abundant element in the universe. If you look at the Sun, you're mostly looking at hydrogen. Its usual ionization color is sort of a purplish-blue. On Earth, it's a transparent gas, which isn't really worth a picture.
Helium - Element 2
 ¿Qué es una Palabra Ecuación en Química?
¿Qué es una Palabra Ecuación en Química?Vuerqex / Public Domain
Helium is the second element on the periodic table and the second most abundant element in the universe. On Earth, it's normally a transparent gas. It can be cooled into a transparent liquid, sort of resembling water, except much, much colder. It ionizes into a reddish orange glowing gas.
Lectura relacionada:
 Cómo Escribir un Informe de Laboratorio
Cómo Escribir un Informe de LaboratorioLithium - Element 3
Lithium in oil.
W. Oelen
Lithium is the third element on the periodic table. This lightweight metal would float on water, but then it would react and burn. The metal oxidizes black in the air. You aren't likely to encounter it in its pure form because it is so reactive.
Beryllium - Element 4
Chinese folding glasses with beryllium lenses, China, mid-18th century.
De Agostini / A. Dagli Orti / Getty Images
The fourth element beryllium. This element is a glossy metal, usually dark from an oxide layer formed by its reaction with air.
Boron - Element 5
Chunks of elemental boron.
James L Marshall
Boron a shiny black metalloid, which means it possesses properties of both metals and nonmetals. Although it can be prepared in a lab, the element does not exist free in nature. It's found in compounds, such as borax.
Carbon - Element #6
The allotropes of carbon, from top: unrefined charcoal, refined charcoal, pressed charcoal, diamonds, and graphite.
Dave King / Getty Images
Most elements can take many forms, called allotropes. Carbon is one of the few elements you can see in daily life as different allotropes. They look quite different from each other and they have distinct properties. Carbon is also important because it's the elemental basis of all organic compounds.
Nitrogen - Element 7
This is the glow given off by ionized nitrogen in a gas discharge tube.
Jurii / Creative Commons
Pure nitrogen is a transparent gas. It forms a transparent liquid and a clear solid that looks much like water ice. However, it's quite colorful as an ionized gas, emitting a blue-violet glow.
Oxygen - Element #8
Liquid oxygen in an unsilvered dewar flask.
Warwick Hillier / Australia National University, Canberra
Pure oxygen is a transparent gas which makes up about 20% of the Earth's atmosphere. It forms a blue liquid. The solid form of the element is even more colorful. Depending on the conditions, it may be blue, red, yellow, orange, or even metallic black!
Fluorine - Element 9
Liquid fluorine.
Prof B. G. Mueller
Fluorine does not occur free in nature, but it can be prepared as a yellowish gas. It cools into a yellow liquid.
Neon - Element 10
This is a photo of a glowing discharge tube filled with neon.
Jurii, Wikipedia Commons
Neon is the first noble gas on the periodic table. The element neon is best known by its reddish orange glow when the element is ionized. Ordinarily, it is a colorless gas.
Sodium - Element 11
Sodium is a soft, silvery reactive metal.
Dnn87 / Creative Commons License
Sodium, like lithium, is a highly reactive metal that will burn in water. The element doesn't occur naturally in pure form, but it's fairly common in science labs. The soft, shiny metal is stored under oil to protect it from oxidation.
Magnesium - Element 12
These are crystals of the pure element magnesium.
Warut Roonguthai
Magnesium is an alkaline earth metal. This reactive metal is used in fireworks. It burns hot enough it can be used to ignite other metals, as in the thermite reaction.
Aluminum - Element 13
Crumpled aluminum foil is a pure form of this common metallic element.
Andy Crawford / Getty Images
Aluminum is a metallic element you often encounter in its pure form, although it requires purification from its ore or else recycling to get it that way.
Silicon - Element 14
This is a photograph of a piece of pure elemental silicon. Silicon is a crystalline metalloid element. Pure silicon is reflective with a dark bluish tinge.
Enricoros / Public Domain
Silicon, like boron, is a metalloid. This element is found in nearly pure form in silicon chips. More commonly, you encounter this element as its oxide in quartz. Although it looks glossy and somewhat metallic, it's too brittle to work like true metals.
Phosphorus - Element 15
The allotropes of phosphorus from left: white phosphorus (yellow cut), red phosphorus, violet phosphorus, and black phosphorus.
BXXXD, Tomihahndorf, Maksim / Materialscientist (Free Documentation License)
Like carbon, phosphorus is a nonmetal that can take any of multiple forms. White phosphorus is deadly toxic and reacts with air to glow green. Red phosphorus is used in safety matches.
Sulfur - Element 16
This picture shows a crystal of pure sulfur.
DEA/A.RIZZI / Getty Images
Sulfur is a nonmetal that can be found in pure form, mostly around volcanoes. The solid element has a distinctive yellow color, but it's red in liquid form.
Chlorine - Element 17
Chlorine gas will condense into a liquid if chilled using dry ice.
Andy Crawford and Tim Ridley / Getty Images
Pure chlorine gas is a noxious greenish-yellow color. The liquid is bright yellow. Like the other halogen elements, it readily reacts to form compounds. While the element can kill you in pure form, it's essential for life. Most of the body's chlorine is ingested as table salt, which is sodium chloride.
Argon - Element 18
This is a 2 cm piece of melting argon ice.
Deglr6328, Free Documentation License
Pure argon gas is transparent. The liquid and solid forms are also colorless. Yet, excited argon ions glow brightly. Argon is used to make lasers, which may be tuned to green, blue, or other colors.
Potassium - Element 19
Like all alkali metals, potassium reacts vigorously in water.
Dorling Kindersley / Getty Images
The alkali metal potassium burns in water, like sodium and lithium, except even more vigorously. This element is one of the ones essential for life.
Calcium - Element 20
Calcium is an alkaline earth metal that oxidizes in air.
Tomihahndorf / Creative Commons License
Calcium is one of the alkaline earth metals. It darkens or oxidizes in air. It is the 5th most abundant element in the body and the most abundant metal.
Scandium - Element 21
These are samples of high purity scandium metal.
Alchemist-hp
Scandium is a lightweight, relatively soft metal. The silver metal develops a yellow or pink tint after exposure to air. The element is used in the production of high intensity lamps.
Titanium - Element 22
This is a bar of high-purity titanium crystals.
Alchemist-hp
Titanium is a light and strong metal used in aircraft and human implants. Titanium powder burns in air and has the distinction of being the only element that burns in nitrogen.
Vanadium - Element 23
This picture shows high purity vanadium in different stages of oxidation.
Alchemist-HP
Vanadium is a shiny gray metal when it's fresh, but it oxidizes in air. The colorful oxidation layer protects the underlying metal from further attack. The element also forms different colored compounds.
Chromium - Element 24
These are crystals of pure elemental chromium metal.
Alchemist-hp, Creative Commons License
Chromium is a hard, corrosion-resistant transition metal. One interesting fact about this element is that the 3+ oxidation state is essential for human nutrition, while the 6+ state (hexavalent chromium) is deadly toxic.
Manganese - Element 25
Mineral nodules of impure manganese metal.
Penny Tweedie / Getty Images
Manganese is a hard, brittle gray transition metal. It's found in alloys and is essential for nutrition, although toxic in high amounts.
Iron - Element 26
This is a photograph of various forms of high-purity elemental iron.
Alchemist-hp / Creative Commons License
Iron is one of the elements you can encounter in pure form in daily life. Cast iron skillets are made of the metal. In pure form, iron is a blue-gray color. It darkens with exposure to air or water.
Cobalt - Element 27
Cobalt is a hard, silvery-gray metal.
Alchemist-hp / Creative Commons License
Cobalt is a brittle, hard metal with an appearance similar to that of iron.
Nickel - Element 28
These are spheres of pure nickel metal.
John Cancalosi / Getty Images
Nickel is a hard, silver metal that can take a high polish. It's found in steel and other alloys. Although it is a common element, it's considered toxic.
Copper - Element 29
This is a sample of native pure copper from Bolivia, South America.
John Cancalosi / Getty Images
Copper is one of the elements you encounter in pure form in daily life in copper cookware and wire. This element also occurs in its native state in nature, meaning you can find copper crystals and chunks. More commonly, it's found with other elements in minerals.
Zinc - Element 30
Zinc is a shiny, corrosion-resistant metal.
Bars Muratoglu / Getty Images
Zinc is a useful metal, found in numerous alloys. It's used to galvanize other metals to protect them from corrosion. This metal is essential for human and animal nutrition.
Gallium - Element 31
Pure gallium has a bright silver color.
Foobar / wikipedia.org
Gallium is considered a basic metal. While mercury is the only liquid metal at room temperature, gallium will melt in the heat of your hand. Even though the element forms crystals, they tend to have a wet, partially melted appearance because of the metal's low melting point.
Germanium - Element 32
Germanium is a hard and lustrous metalloid or semimetal.
Jurii
Germanium is a metalloid with an appearance similar to that of silicon. It's hard, shiny, and metallic in appearance. The element is used as a semiconductor and for fiberoptics.
Arsenic - Element 33
The gray form of arsenic may take the form of interesting-looking nodules.
Harry Taylor / Getty Images
Arsenic is a poisonous metalloid. It sometimes occurs in the native state. Like other metalloids, it takes multiple forms. The pure element may be a gray, black, yellow, or metallic solid at room temperature.
Selenium - Element 34
Like many nonmetals, pure selenium exists in markedly different forms.
W. Oelen / Creative Commons
You can find the element selenium in dandruff-control shampoos and some types of photographic toner, but it's not commonly encountered in pure form. Selenium is a solid at room temperature and takes red, gray, and metallic-looking black forms. They gray allotrope is most common.
Bromine - Element 35
This is a picture of the element bromine in a vial encased in a block of acrylic.
Alchemist-hp / Creative Commons License
Bromine is a halogen that is a liquid at room temperature. The liquid is deep reddish-brown and vaporizes into an orange-brown gas.
Krypton - Element 36
This is a photo of the element krypton in a gas discharge tube.
Alchemist-hp
Krypton is one of the noble gases. A picture of krypton gas would be pretty boring, because it basically looks like air (which is to say, it's colorless and transparent). Like other noble gases, it lights up colorfully when ionized. Solid krypton is white.
Rubidium - Element 37
This is a sample of pure liquid rubidium metal.
Dnn87, Free Documentation License
Rubidium is a silver-colored alkali metal. Its melting point is only slightly higher than room temperature, so it may be observed as a liquid or soft solid. However, it's not a pure element you'd want to handle, since it ignites in air and water, burning with a red flame.
Strontium - Element 38
These are crystals of the pure element strontium.
Alchemist-HP
Strontium is a soft, silver alkaline earth metal that develops a yellowish oxidation layer. You probably won't ever see this element in its pure form except in pictures, but it's used in fireworks and emergency flares for the bright red color it adds to flames.
Yttrium - Element 39
Yttrium is a silvery metal.
Alchemist-hp
Yttrium is a silver-colored metal. It's fairly stable in air, although it will eventually darken. This transition metal is not found free in nature.
Zirconium - Element 40
Zirconium is a gray transition metal.
Alchemist-hp
Zirconium is a lustrous gray metal. It's known for its low neutron absorption cross-section, so it's an important element in nuclear reactors. The metal is also known for its high corrosion resistance.
Niobium - Element 41
Niobium is a bright silver metal that develops a metallic blue color over time in air.
Alchemist-hp
Fresh, pure niobium is a bright platinum-white metal, but after exposure in the air it develops a blue cast. The element is not found free in nature. It's usually associated with the metal tantalum.
Molybdenum - Element 42
These are examples of pure molybdenum metal.
Alchemist-hp
Molybdenum is a silvery-white metal belonging to the chromium family. This element is not found free in nature. Only the elements tungsten and tantalum have higher melting points. The metal is hard and tough.
Ruthenium - Element 44
Ruthenium is a very hard, silver-white transition metal.
Periodictableru
Ruthenium is another hard white transition metal. It belongs to the platinum family. Like other elements in this group, it resists corrosion. This is good, because its oxide has a tendency to explode in air!
Rhodium - Element 45
These are different forms of pure elemental rhodium.
Alchemist-HP
Rhodium is a silvery transition metal. Its primary use is as a hardening agent for softer metals, such as platinum and palladium. This corrosion-resistant element is also considered a noble metal, like silver and gold.
Silver - Element 47
This is a crystal of pure silver metal.
Gary Ombler / Getty Images
Silver is a silver-colored metal (hence the name). It forms a black oxide layer called tarnish. While you may be familiar with the appearance of silver metal, you might not realize the element also forms beautiful crystals.
Cadmium - Element 48
This is a photo of a cadmium crystal bar and a cube of cadmium metal.
Alchemist-hp, Creative Commons License
Cadmium is a soft, blue-white metal. It's primarily used in soft and low melting point alloys. The element and its compounds are toxic.
Indium - Element 49
Indium is an extremely soft, silver-white metal.
Nerdtalker
Indium is a post-transition metallic element that has more in common with the metalloids than with the transition metals. It is very soft with a silver metallic luster. One of its interesting properties is that the metal wets glass, making it an excellent material for making mirrors.
Tin - Element 50
This image shows the two allotropes of the element tin, white and grey tin.
Alchemist-HP
You are familiar with the shiny metallic form of tin from tin cans, but colder temperatures change the allotrope of the element into gray tin, which doesn't behave like a metal. Tin is commonly applied over other metals to help protect them from corrosion.
Tellurium - Element 52
This is a picture of pure tellurium metal. The sample is 3.5 cm across.
Tellurium one of the metalloids or semimetals. It occurs in either a shiny gray crystalline form or else a brownish-black amorphous state.
Iodine - Element 53
At room temperature and pressure, iodine occurs as either a violet solid or vapor.
Matt Meadows / Getty Images
Iodine is another element that displays a distinctive color. You might encounter it in a science lab as a violet vapor or as a shiny blue-black solid. The liquid does not occur at normal pressure.
Xenon - Element 54
This is a sample of pure liquid xenon.
Rasiel Suarez on behalf of Luciteria LLC
The noble gas xenon is a colorless gas under ordinary conditions. Under pressure, it may be liquefied into a transparent liquid. When ionized, the vapor emits pale blue light.
Europium - Element 63
This is a photo of pure europium.
Alchemist-hp, Creative Commons License
Europium is a silver metal with a slight yellow tint, but it oxidizes instantly in air or water. This rare earth element actually is rare, at least in the universe where it's estimated to have an abundance of 5 x 10-8 percent of matter. Its compounds are phosphorescent.
Thulium - Element 69
This is a picture of forms of elemental thulium.
Alchemist-hp, Creative Commons License
Thulium is the rarest of the rare earths (which are actually fairly abundant overall). Because of this, there aren't many uses for this element. It's not toxic, but doesn't serve any known biological function.
Lutetium - Element 71
Lutetium, like other rare earth elements, does not occur in pure form in nature.
Alchemist-hp / Creative Commons License
Lutetium is a soft, silvery rare earth metal. This element does not occur free in nature. It's used primarily for catalysts in the petroleum industry.
Tantalum - Element 73
Tantalum is a lustrous blue-gray transition metal.
Alchemist-hp
Tantalum is a shiny blue-gray metal often found in association with the element niobium (located right above it on the periodic table). Tantalum is highly resistant to chemical attack, although it is affected by hydrofluoric acid. The element has an extremely high melting point.
Tungsten - Element 74
Tungsten is a brittle metal, though it has an extremely high tensile strength.
Alchemist-hp
Tungsten is a strong, silver-colored metal. This is the element with the highest melting point. At high temperatures, a colorful oxidation layer can form over the metal.
Osmium - Element 76
Osmium is a brittle and hard blue-black transition metal. This cluster of osmium crystals was grown using chemical vapor transport.
Periodictableru
Osmium is a hard, shiny transition metal. Under most conditions, it is the element with the highest density (about twice as heavy as lead).
Platinum - Element 78
Platinum is a dense, grayish-white transition metal.
Periodictableru, Creative Commons License
The metal platinum is seen in relatively pure form in high-end jewelry. The metal is heavy, fairly soft, and corrosion resistant.
Gold - Element 79
This is a nugget of pure gold.
Harry Taylor / Getty Images
Element 79 is the precious metal, gold. Gold is known by its distinctive color. This element, along with copper, are the only two non-silvery metals, although it is suspected some of the new elements may display colors (if enough is ever produced to see them).
Mercury - Element 80
Mercury is the only metal that is a liquid at room temperature and pressure.
Harry Taylor / Getty Images
Mercury also goes by the name quicksilver. This silver-colored metal that is a liquid at room temperature and pressure. You may be wondering what mercury looks like when it is solid. Well, if you place a bit of mercury in liquid nitrogen, it will solidify into a gray metal that resembles tin.
Thallium - Element 81
These are chunks of pure thallium sealed in an ampoule with argon gas.
W. Oelen
Thallium is a soft, heavy post-transition metal. The metal resembles tin when it's fresh, but discolors to blue-gray upon exposure to air. The element is soft enough to cut with a knife.
Lead - Element 82
Lead darkens in air, although the pure metal is silver-colored.
Alchemist-hp
Element 82 is lead, a soft, heavy metal best known for its ability to shield against x-rays and other radiation. The element is toxic, yet common.
Bismuth - Element 83
The crystal structure of the metal bismuth is as beautiful as the oxide layer that forms on it.
Kerstin Waurick / Getty Images
Pure bismuth is a silver-gray metal, sometimes with a faint pink tinge. However, this element readily oxidizes into a rainbow array of colors.
Uranium - Element 92
This is a lump of uranium metal recovered from a Titan II missile.
Martin Marietta; Roger Ressmeyer/Corbis/VCG / Getty Images
Uranium is a heavy, radioactive metal belonging to the actinide group. In pure form, it's a silver-gray metal, able to take a high polish, but it accumulates a dull oxidation layer after exposure to air.
Plutonium - Element 94
Plutonium is a silvery-white radioactive metal.
U.S. Department of Energy
Plutonium is a heavy radioactive metal. When fresh, the pure metal is shiny and silver. It develops a yellowish oxidation layer after exposure to air. It's unlikely you'll ever get a chance to view this element in person, but if you do, turn out the lights. The metal appears to glow red.