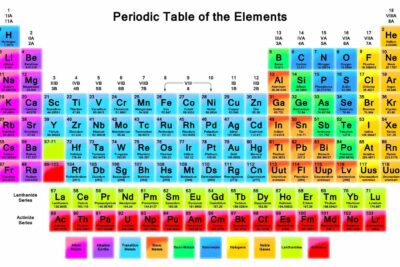
One reason the periodic table of the elements is so useful is that it is a means of arranging elements according to their similar properties. This is what is meant by periodicity or periodic table trends.
There are multiple ways of grouping the elements, but they are commonly divided into metals, semimetals (metalloids), and nonmetals. You'll find more specific groups, like transition metals, rare earths, alkali metals, alkaline earth, halogens, and noble gasses.
Lectura relacionada:
 Proyección de Cuña y Tablero: Definición y Ejemplo
Proyección de Cuña y Tablero: Definición y EjemploGroups in the Periodic Table of Elements
Click on an element to read about the chemical and physical properties of the group to which that element belongs.
Alkali Metals
- Less dense than other metals
- One loosely bound valence electron
- Highly reactive, with reactivity increasing moving down the group
- The largest atomic radius of elements in their period
- Low ionization energy
- Low electronegativity
Lectura relacionada:
 4 Tipos y Ejemplos de Meteorización Química
4 Tipos y Ejemplos de Meteorización QuímicaAlkaline Earth Metals
- Two electrons in the valence shell
- Readily form divalent cations
- Low electron affinity
- Low electronegativity
Transition Metals
The lanthanides (rare earth) and actinides are also transition metals. The basic metals are similar to transition metals but tend to be softer and to hint at nonmetallic properties. In their pure state, all of these elements tend to have a shiny, metallic appearance. While there are radioisotopes of other elements, all of the actinides are radioactive.
- Very hard, usually shiny, ductile, and malleable
- High melting and boiling points
- High thermal and electrical conductivity
- Form cations (positive oxidation states)
- Tend to exhibit more than one oxidation state
- Low ionization energy
Lectura relacionada:
 Nombres y Usos de Cristalería de Laboratorio
Nombres y Usos de Cristalería de LaboratorioMetalloids or Semimetals
- Electronegativity and ionization energy intermediate between that of metals and nonmetals
- May possess a metallic luster
- Variable density, hardness, conductivity, and other properties
- Often make good semiconductors
- Reactivity depends on the nature of other elements in the reaction
Nonmetals
The halogens and noble gases are nonmetals, although they have their own groups, too.
- High ionization energy
- High electronegativity
- Poor electrical and thermal conductors
- Form brittle solids
- Little if any metallic luster
- Readily gain electrons
Halogens
The halogens exhibit different physical properties from each other but do share chemical properties.
- Extremely high electronegativity
- Very reactive
- Seven valence electrons, so elements from this group typically exhibit a -1 oxidation state
Noble Gases
The noble gasses have complete valence electron shells, so they act differently. Unlike other groups, noble gasses are unreactive and have very low electronegativity or electron affinity.
Periodic Table of Element Groups
Click on the element symbol in the table for further information.
| 1 IA 1A |
18 VIIIA 8A |
||||||||||||||||
| 1 H 1.008 |
2 IIA 2A |
13 IIIA 3A |
14 IVA 4A |
15 VA 5A |
16 VIA 6A |
17 VIIA 7A |
2 He 4.003 |
||||||||||
| 3 Li 6.941 |
4 Be 9.012 |
5 B 10.81 |
6 C 12.01 |
7 N 14.01 |
8 O 16.00 |
9 F 19.00 |
10 Ne 20.18 |
||||||||||
| 11 Na 22.99 |
12 Mg 24.31 |
3 IIIB 3B |
4 IVB 4B |
5 VB 5B |
6 VIB 6B |
7 VIIB 7B |
8 ← ← |
9 VIII 8 |
10 → → |
11 IB 1B |
12 IIB 2B |
13 Al 26.98 |
14 Si 28.09 |
15 P 30.97 |
16 S 32.07 |
17 Cl 35.45 |
18 Ar 39.95 |
| 19 K 39.10 |
20 Ca 40.08 |
21 Sc 44.96 |
22 Ti 47.88 |
23 V 50.94 |
24 Cr 52.00 |
25 Mn 54.94 |
26 Fe 55.85 |
27 Co 58.47 |
28 Ni 58.69 |
29 Cu 63.55 |
30 Zn 65.39 |
31 Ga 69.72 |
32 Ge 72.59 |
33 As 74.92 |
34 Se 78.96 |
35 Br 79.90 |
36 Kr 83.80 |
| 37 Rb 85.47 |
38 Sr 87.62 |
39 Y 88.91 |
40 Zr 91.22 |
41 Nb 92.91 |
42 Mo 95.94 |
43 Tc (98) |
44 Ru 101.1 |
45 Rh 102.9 |
46 Pd 106.4 |
47 Ag 107.9 |
48 Cd 112.4 |
49 In 114.8 |
50 Sn 118.7 |
51 Sb 121.8 |
52 Te 127.6 |
53 I 126.9 |
54 Xe 131.3 |
| 55 Cs 132.9 |
56 Ba 137.3 |
* | 72 Hf 178.5 |
73 Ta 180.9 |
74 W 183.9 |
75 Re 186.2 |
76 Os 190.2 |
77 Ir 190.2 |
78 Pt 195.1 |
79 Au 197.0 |
80 Hg 200.5 |
81 Tl 204.4 |
82 Pb 207.2 |
83 Bi 209.0 |
84 Po (210) |
85 At (210) |
86 Rn (222) |
| 87 Fr (223) |
88 Ra (226) |
** | 104 Rf (257) |
105 Db (260) |
106 Sg (263) |
107 Bh (265) |
108
(265) |
109 Mt (266) |
110 Ds (271) |
111 Rg (272) |
112 Cn (277) |
113 Uut -- |
114 Fl (296) |
115 Uup -- |
116 Lv (298) |
117 Uus -- |
118 Uuo -- |
| * Lanthanide Series |
57 La 138.9 |
58 Ce 140.1 |
59 Pr 140.9 |
60 Nd 144.2 |
61 Pm (147) |
62
150.4 |
63 Eu 152.0 |
64 Gd 157.3 |
65 Tb 158.9 |
66 Dy 162.5 |
67 Ho 164.9 |
68 Er 167.3 |
69 Tm 168.9 |
70 Yb 173.0 |
71 Lu 175.0 |
| ** Actinide Series |
89 Ac (227) |
90 Th 232.0 |
91 Pa (231) |
92 U (238) |
93 Np (237) |
94 Pu (242) |
95 Am (243) |
96 Cm (247) |
97 Bk (247) |
98 Cf (249) |
99 Es (254) |
100 Fm (253) |
101 Md (256) |
102 No (254) |
103 Lr (257) |